Want to know more about this article?
Recyclable bioplastic membrane to clear oil spills from water
Polymer scientists from the University of Groningen and NHL Stenden University of Applied Sciences, both in the Northern Netherlands, have developed a polymer membrane from biobased malic acid. It is a membrane that can be used to separate water and oil, and it is fully recyclable. When the pores are blocked by foulants, it can be depolymerized, cleaned and subsequently pressed into a new membrane.
How do you clean up an oil spill in water? This is quite a challenge. Superamphiphilic membranes, that ‘love’ both oil and water, are a promising solution but not yet a very practical one. These membranes are often not robust enough for use outside the laboratory environment and the membrane pores can clog up as a result of fouling by algae and sand.
Chongnan Ye and Katja Loos from the University of Groningen and Vincent Voet and Rudy Folkersma from NHL Stenden used a relatively new type of polymer to create a membrane that is both strong and easy to recycle.
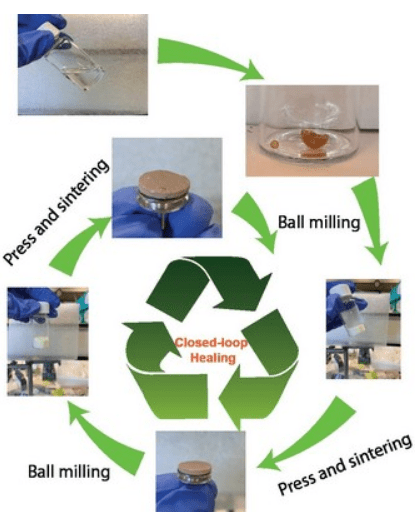
The new vitrimer membrane is made by pressing and sintering of polymers from the natural monomer malic acid. This membrane can be recycled by ball milling and subsequent pressing and sintering. | Illustration: Chongnan Ye, University of Groningen
Dynamic network
In recent years, the researchers from both institutes have joined forces to investigate vitrimer plastics, polymer materials that have the mechanical properties and chemical resistance of a thermoset plastic. However, vitrimer plastics can also behave like a thermoplastic, since they can be depolymerized and reused. This means that a vitrimer plastic has all the qualities to make a good membrane for oil spill remediation. ‘Furthermore, it was made from malic acid, a natural monomer,’ adds Loos.
‘The polymers in the vitrimer are crosslinked in a reversible manner,’ explains Voet. ‘They form a dynamic network, which enables recycling of the membrane.’ The vitrimer is produced through base-catalysed ring-opening polymerization between pristine and epoxy-modified biobased malic acid. The polymers are ground into a powder by ball milling and turned into a porous membrane through the process of sintering.
Pores
Both water and oil will spread out on the resulting superamphiphilic membrane. In an oil spill, much more water is present than oil, which means that the membrane is covered by water that can then pass through the pores. Voet: ‘The water film on the membrane’s surface keeps the oil out of the pores so that it is separated from the water.’
The membrane is firm enough to filter oil from the water. When sand and algae clog up the pores, the membrane can be depolymerized and recreated from the building blocks after removal of the pollutants. ‘We have tested this on a laboratory scale of a few square centimetres,’ says Loos. ‘And we are confident that our methods are scalable, both for the polymer synthesis and for the production and recycling of the membrane.’ The scientists are hoping that an industrial partner will take up further development.
Applications
Creating this new membrane for oil spill remediation shows the power of cooperation between a research university and an applied university. ‘A while ago, we decided that the polymer groups at the two institutes should become one, by sharing students, staff and facilities. We recently started the first hybrid research group in the Netherlands,’ explains Loos. This makes it easier to find applications for newly designed materials. Voet: ‘Polymer chemists strive to link molecular structures to material properties and applications. Our hybrid research team has the experience to do just that.’
Story Source:
Materials provided by University of Groningen. Note: Content may be edited for style and length.
A paper describing the creation of this membrane was published in the journal Advanced Materials on 7 March 2021.